Deep Down In The Abyss: The prehistoric 'Sea Monster' of the Mesozoic era
Introduction 'She sells seashells by the seashore'. This tongue twister was based on Mary Anning, a pioneering palaeontologist and fossil collector (Strauss, 2019). In the early 19th century, she was the first to discover what is now named Ichthyosaurus communis, meaning 'fish-lizard' (Mary-Claire Eylott, n.d). However, this genus was neither a fish nor a lizard. Scientists classify Ichthyosaurus communis as a marine reptile (Marek, 2015). Ichthyosaurus communis is a part of the order Ichthyosauria, which sits in the superorder of Ichthyopterygia (Fig.1). Ichthyosauria holds many records: possessing the largest eyes out of all living things ever to have existed (Motani, Rothschild and Wahl, 1999), being the first air breathing tetrapods with a fish-like body (Marek, 2015) and even laying claim to being the largest animal on earth of all time (Lomax et al., 2018).
Evolutionary History The history of all life started int he waters. Some of the living species acquired lungs and were able to breathe air above the surface of water. Eventually these species were able to exit the seas and live on land. However, these early terrestrial dwellers re-entered the waters. Most likely due to the competition on land. It is these land dwellers which are thought to be the ancestry of the Ichthyosauria. Thus, the ichthyosaurs were known to be the secondary aquatic animals (Lomax, 2018; Marek, 2015). Ichthyosauria ruled most of the Mesozioc era (Motani, 2009: p.7). From the Early Triassic to Late Cretaceous, around 250 millions years to 90 million years ago, there were three evolutionary grades of Ichthyosauria (Moon, 2017: p.36).
Grade I: basal The basal grade of ichthyosaurs encompassed all the early undeveloped forms. It was in the early Triassic when a huge burst in diversity occurred within the Ichthyosauria (Sander, 2021). The 3 focal early genera were Chaohusaurus from China, Utatsusaurus from Japan and Grippia from Canada. All these early forms ranged from 1 to 3 metres in length (Lomax, 2018). Every genera had a long narrow body, a small caudal fin and a high presacral vertebrae count. These features pointed towards an anguilliform swimming mode (Motani et al., 1996). The swimming mode is similar to how modern-day eels locomote. The basal ichthyosaurs wiggled their long narrow bodies to move around. Due to this swimming mode these early forms could not swim fast, deep or long distances (Marek, 2015).
Grade II: intermediate A few million years later, during the mid-Triassic, the species Mixosaurus cornalianus, developed 5 distinct rows of finger bones in the front paddle (Bassani, 1886). In addition, the Mixosaurus genus was also the first to show evidence of vivaparity in the Ichthyosauria (Motani, 2009: p. 9). Essentially vivaparity signified that ichthyosaurs gave birth to live young. Since the ichthyosaurs had their fins modified, they were unable to walk on land (Lomax, 2018). Consequently, they had to stay in the waters. The isolation from terrain prevented the marine reptiles from laying eggs on land. As a result the intermediate grade of Ichthyosauria adapted and gave birth to live young, without having to exit the water. A fossil from Holzmaden, Germany of a female Stenopterygius quadriscissus clearly shows 3 unborn young inside her body. A fourth baby is seen to come out of the mother tail first. The reason for the young to exit tail first was so that they could have time to acclimate and to ultimately prevent themselves from drowning (Strauss, 2019). Another adaptation the intermediate grade acquired was the ability to engage in hemispheric sleep. Hemispheric sleep describes one side of the brain resting at a time. As the ichthyosaurs were always in water, hemispheric sleep allowed them to be constantly on the move (Motani, 2009: p. 8). Not too long before the Triassic - Jurassic boundary, approximately 210 million years ago, a fourth mass extinction event had occurred. Close to 76% of all land and aquatic species went extinct, while the order Ichthyosauria survived (Britannica, 2021). Nevertheless, the vast diversity that was present in the Triassic stagnated and started to plummet. Only 3 to 4 lineages remained. This fragment in time was known as the evolutionary bottleneck (Thorne et al., 2011).
Grade III: fish shaped In the early Jurassic, approximately 201 million years ago, the ichthyosaur genera evolved even further by changing their appearance to look like the stereotypical version of the Ichthyosaur images we observe online. Parvipalvia, meaning ‘small pelvic girdle’ was the main group that demonstrated this iconic appearance (Motani, 2009: p.7). The fish-shaped grade of Ichthyosauria adopted a new thunniform body plan. Namely, a fusiform (streamlined) body shape, long thin snout, and paired flipper-like appendages (Motani et al., 1996). The eye size grew larger in conjunction with the body (Motani, 2009: p.8). In addition to this the new fish shaped forms evolved discoidal vertebrae, retaining their high vertebrae count. They also gained highly modified dorsal and tail fins, which the early forms didn’t possess. These new ‘upgrades’ in the body plan indicated a pelagic swimming mode (Motani et al., 1996). Ichthyosaurs moved their crescent-like tail fin side to side in order to swim faster and greater distances than their earlier forms. The paired appendages were most likely present for maneuvering while
swimming at great speeds, approximately 21Mph (Lomax, 2018). Scientists and collectors also noticed that the lower lobe of the vertebral column only went down into the ventral part of the tail fin, leaving the dorsal part with no hard tissue (Motani, 2009: p. 7). Hence, the dorsal part is not commonly found in fossils. As well as being agile swimmers, Jurassic Ichthyosaurs were commendable divers. Ichthyosaurs could swim down to about 600m deep. The Jurassic forms adapted for deep dives by growing bigger eyes and having their skeleton lightened (Marek, 2015). Evidence for this can be found by looking at the absurdly large eye sockets. It was estimated that the largest eyes were greater than 25cm in diameter (Motani, 2009: p.7), which is bigger than a soccer ball. The colossal eyes could absorb a large amount of light indicating that the Ichthyosaurs could have roamed in low light environments. Another piece of evidence is Caisson disease, colloquially known as ‘the bends’ (Marek, 2015). The Caisson disease was caused from swimming up too quickly from a great depth causing the body to experience a drastic change in pressure. This disease was identified in a number of ichthyosaurs by finding gas bubbles on the interior. As ichthyosaurs were air breathing tetrapods, they had to resurface in order to fill their system with oxygen. Ichthyosaurs that swam up too quickly in order to escape a predator or because they urgently needed more air would have received this disease. During the Early Cretaceous there was only a single genus called Platypterigius. Very few Ichthyosauria fossils were found in the Cretaceous beds, showing a massive drop in diversity and a path to ichthyosaur extinction (Fischer et al., 2014). Ichthyosaurs disappeared from the fossil record in the Late Cretaceous, about 90 million years ago, 25 million years before the 5th mass extinction when all the dinosaurs were wiped off planet earth (Bardet, 1992). There is no definitive answer as to how the ichthyosaur empire ceased to exist. Dale (2018) and Fischer et al. (2016) hypothesise that there was a great ocean anoxic event. The rest of the majority propose it was the steady decline in diversity and numbers that led to ichthyosaur extinction. Even though the ichthyosaurs became extinct they lived the 2nd most out of all the marine reptiles during the Mesozoic (Fig.3) implying that the Ichthyosauria were a hugely successful clade of marine reptiles (Motani, 2009: e4).Homology and Homoplasy in the Ichthyosauria Both homology and homoplasy are single anatomical features of species in a phylogeny. Homology concerns itself with traits that are derived from a single common ancestor.
Forefin The order Ichthyosauria have paddle like limbs, known as forefins. Those limbs were greatly modified throughout the evolution of the Ichthyosauria. The Jurassic period was undoubtedly the one in which forefin morphology was extremely diverse and complex. An example of a homologous feature within the Ichthyosauria is owning an extra zeugopodial element anterior to the radius and in contact with the humerus. Genera such as Ophthalmosaurus, Brachypterygius, Caypullisaurus and Platypterigius all share this feature in the forefin (Motani, 1999: p. 39; Fernandez, 2010: p.517). This zeugopodial element is seen to be in contact with the humeri of all species mentioned prior. Furthermore the different elementsin contact with the humerus is another homologous feature. There are 3 different types of contact. No.1, the humerus is in contact with the radius, ulna and extra zeugopodial element. This first type of contact is seen in Ophthalmosaurus, Caypullisaurus and in the species Platypterigius longmani. No.2, the humerus is in contact with the radius, ulna and intermedium. This contact with the humerus can be seen in Brachypterygius and Aegirosaurus. No.3, the humerus contacts the same radius, ulna, extra zeugopodial element as well as a pisiform. Most Tithonian ichthyosaurs have this type of contact (Fernandez, 2001: p. 519).
Homoplasy concerns itself with features of species not derived from a single common ancestor. The order Ichthyosauria provides us with many cases of homoplasy. For instance, the different skin layers of an Ichthyosaur. Soft parts are not preserved as well as hard parts and so are a rare find in the fossil record. However, an unusual preservation of Stenopterygius soft tissue remnants were discovered in Holzmaden, Germany dating back 178 million years ago. Those same tissue remnants provided us with many homoplasy examples in the Ichthyosauria.
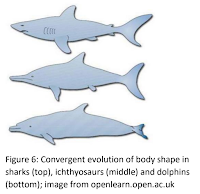
Skin shading From analysing the soft tissue remnants, traces of pigment carrying cells known as chromatophores were found (Black, 2018). Namely, melanophores containing the pigment melanin (Greshko, 2018). Scientists used chromatophores to explore what colours the ichthyosaur possessed and uncovered that adult species showed countershading. Countershading is a camouflaging technique. Ichthyosaurs had a dark shading on the dorsal side and light shading on the ventral side. Thus, the dark side would blend in with the dark water from the above perspective and the light side would mix in with the bright sky from below (Lindgren et al., 2018; Buchholz, n.d.). The order Lamniformes to which the mackerel sharks belong also show countershading. Dolphins, in the order Cetacea, also exhibit the same colouration pattern of the outer skin. Even though sharks, dolphins and ichthyosaurs have some similarity in their overall appearance, as seen in (Fig.6), they do not have a common ancestor.
Blubber After describing the homoplasy of the outer skin it made sense to do the same thing on the interior. Blubber is a fatty layer deep beneath the skin. This fatty layer was found in the Stenopterygius at Holzmaden. The possession of this insulating layer ties ichthyosaurs to elevated metabolism, implying that they were endotherms (Black, 2018; Greshko,2018). This meant that ichthyosaurs were able to stay warmer than the surrounding environment, which would explain how they could swim in cold deep waters. A different group are also known for their internal body heating. The order Cetacea in which dolphins, porpoises, whales lie (Black, 2018). The extinct Ichthyosauria and extant Cetacea do not have the same ancestor. However, they independently evolved similarities on the inside.Scientific significance of Ichthyosaurus communis in the order Ichthyosauria Ichthyosaurus communis from Greek translates to common fish lizard. Furthermore, I. communis is the type species for the genus Ichthyosaurus, which in turn is the type genus for the order Ichthyosauria (Lomax et al., 2017; Massare and Lomax, 2017). That is the primal reason why the Ichthyosauria is named as it is. The total amount of species within the genus was over 50, when new ichthyosaur fossils were found every day. At present Ichthyosaurus has stopped being the ‘wastebasket’ for all found ichthyosaurs and now only holds 6 species. The method used for redefining and categorising the species, involved using the neotype of Ichthyosaurus communis (Massare and Lomax, 2017). In the remote past the neotype of I. communis was thought to be the neotype of I. intermedius. However, McGowan (1974) suggests that both I. communis and I. intermedius can be identified as one species, as the majority of the features in both neotypes, of each species, are similar. And since the name I. communis was announced earlier it held the priority. The original stratigraphic range for I. communis was between the Rhaetian and Sinemurian (Bennett, 2012). This changed when a new specimen of Ichthyosaurus communis [NHMUK R15907] was found in Stonebarrow Marl at Charmouth, Dorset.
Conclusion This research on the order of Ichthyosauria highlights the evolutionary history of the group. Specifically how the underdeveloped basal forms with unpronounced appendages and tail evolved a fish-like body plan and modified its limbs. The forefin was the source of most homologous traits due to its highly modified elements and diversity within the group. The similarity and convergent evolution between the Lamniformes, Cetacea and Icthyopterygia was discussed. And incontrovertibly, Ichthyosaurus communis demonstrated many points on why it is significant to the scientific community as the type species of the order Ichthyosauria.
Bibliography Bardet, N., 1992. Stratigraphic evidence for the extinction of the ichthyosaurs. [ebook] Available at: <https://onlinelibrary.wiley.com/doi/epdf/10.1111/j.1365- 3121.1992.tb00614.x?saml_referrer>.
Bennett et al., S., 2012. A new specimen of Ichthyosaurus communis from Dorset, UK, and its bearing on the stratigraphical range of the species. [ebook] Available at: <https://www.sciencedirect.com/science/article/pii/S0016787811000678#!>.
Black, R., 2018. Like whales and dolphins prehistoric 'fish lizards' kept warm with blubber. Smithsonian, [online] Available at: <https://www.smithsonianmag.com/science-nature/whales-and-dolphins- prehistoric-fish-lizards-kept-warm-blubber-180970958/>.
Buchholz, P., n.d. Dolphin-like marine reptiles were more dolphin-like than previously thought. [ebook] Available at: <https://eartharchives.org/articles/dolphin-like- marine-reptiles-were-more-dolphin-like-than-previously-thought/index.html>.
Dale, W., 2018. Response of Cretaceous marine reptiles to palaeoceanographic changes: sea-level and climate changes as drivers of origination and extinction. [ebook] Available at: <https://etd.ohiolink.edu/apexprod/rws_olink/r/1501/10?clear=10&p10_accessio n_num=bgsu1542284081403242>.
Dick et al., D., 2016. Trophic niche ontogeny and palaeoecology of early Torcian Stenopterygius (Reptilia: Ichthyosauria). [ebook] Available at: <https://onlinelibrary.wiley.com/doi/10.1111/pala.12232>.
Eylott, M., n.d. Mary Anning: the unsung hero of fossil discovery. [online] Nhm.ac.uk. Available at: <https://www.nhm.ac.uk/discover/mary-anning-unsung-hero.html>.
Fernandez, M., 2001. Dorsal or Ventral? Homologies of the forefin Caypullisaurus (Ichthyosauria: Ophthalmosauria). [ebook] Available at: <https://www.tandfonline.com/doi/pdf/10.1671/0272- 4634%282001%29021%5B0515%3ADOVHOT%5D2.0.CO%3B2?needAccess=t rue>.
Fischer et al., V., 2014. High diversity in Cretaceous ichthyosaurs from Europe prior to their extinction. [ebook] Available at: <https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0084709>.
Fischer et al., V., 2016. Extinction of fish-shaped marine reptiles associated with reduced rates and global environmental volatility. [ebook] Available at: <https://www.nature.com/articles/ncomms10825>.
Greshko, M., 2018. Incredible 'sea monster' fossil still has skin and blubber. National Geographic, [online] Available at: <https://www.nationalgeographic.com/science/article/incredible-jurassic- ichthyosaur-fossil-preserves-skin-blubber>.
Lindgren et al., J., 2018. Soft-tissue evidence for homeothermy and crypsis in a Jurassic ichthyosaur. [ebook] Available at: <https://www.nature.com/articles/s41586-018-0775-x>.
Lomax, D., 2018. The evolutionary history of ichthyosaurs. [ebook] Available at: <https://beta.capeia.com/paleobiology/2017/09/25/the-evolutionary-history-of- ichthyosaurs>.
Lomax et al., D., 2017. The first known neonate Ichthyosaurus communis skeleton: a rediscovered specimen from the Lower Jurassic, UK. [ebook] Available at: <https://www.tandfonline.com/doi/full/10.1080/08912963.2017.1382488>.
Lomax et al., D., 2018. A giant Late Triassic ichthyosaur from the UK and the reinterpretation of the Aust cliff 'dinosaurian' bones. [ebook] Available at: <https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0194742>.
Maisch, M., 2000. The Ichthyosauria. [ebook] Available at: <https://onlinelibrary.wiley.com/doi/10.1111/pala.12232>.
Marek, R., 2015. PALAEONTOLOGY[online] | Article: Fossil Focus > Fossil Focus: Ichthyosaurs. [online] PALAEONTOLOGY[online]. Available at: <https://www.palaeontologyonline.com/articles/2015/fossil-focus- ichthyosaurs/?doing_wp_cron=1636379956.8781421184539794921875>.
Massare, J. and Lomax, D., 2017. A taxonomic reassessment of Ichthyosaurus communis and I. intermedius and a revised diagnosis for the genus. [ebook] Available at: <https://www.tandfonline.com/doi/full/10.1080/14772019.2017.1291116>.
Maxwell, E., 2013. An evolutionary and developmental perspective on the loss of regionalization in the limbs of derived ichthyosaurs. [ebook] Available at: <https://www.cambridge.org/core/journals/geological-magazine/article/an- evolutionary-and-developmental-perspective-on-the-loss-of-regionalization-in- the-limbs-of-derived-ichthyosaurs/4BC483114A27C34E1C2E26F7AEEAD526>.
McGowan, C., 1974. A revision of the latipinnate ichthyosaurs of the lower jurassic of England (Reptilia: Ichthyosauria). Toronto: Royal Ontario Museum.
Moon, B., 2017. A new phylogeny of ichthyosaurs (Reptilia: Diapsida). [ebook] Available at: <https://research- information.bris.ac.uk/ws/portalfiles/portal/142491822/Typescript_A_new_phylog eny_of_ichthyosaurs.pdf>.
Motani, R., 1999. On the evolution and homologies of Ichthyopterygian forefins. [ebook] Available at: <https://www.jstor.org/stable/4523967?seq=1#metadata_info_tab_contents>.
Motani, R., 1999. Phylogeny of the Ichthyopterygia. [ebook] Available at: <https://web.archive.org/web/20160305104153/http://mygeologypage.ucdavis.ed u/motani/pdf/Motani1999c.pdf>.
Motani, R., 2009. The Evolution of Marine Reptiles. [ebook] Available at: <https://link.springer.com/content/pdf/10.1007/s12052-009-0139-y.pdf>.
Motani et al., R., 1996. Eel-like swimming in the earliest ichthyosaurs. [ebook] Available at: <https://www.nature.com/articles/382347a0>.
Motani, R., Rothschild, B. and Wahl, W., 1999. Large eyeballs in diving ichthyosaurs. [ebook] Available at: <https://www.nature.com/articles/45435>.
Sander, M., 2021. Ichthyosauria. In: M. Sander, ed., Vertebrate Skeletal Histology and Palaeohistology, 1st ed. [online] Available at: <https://www.taylorfrancis.com/chapters/edit/10.1201/9781351189590- 24/ichthyosauria-martin-sander>.
Strauss, B., n.d. Learn About the Prehistoric Marine Reptile Ichthyosaurus. [online] ThoughtCo. Available at: <https://www.thoughtco.com/ichthyosaurus-1091502> [Accessed 13 November 2019].
Thorne et al., P., 2011. Resetting the evolution of marine reptiles at the Triassic- Jurassic boundary. [ebook] Available at: <https://www.pnas.org/content/108/20/8339>.







Comments
Post a Comment